Difference between revisions of "Gene function"
Farmakorakel (talk | contribs) |
Farmakorakel (talk | contribs) (→Description of problem) |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | Next generation sequencing ([[NGS]]) leads to the discovery of many new genetic variants, as outlined | + | Next generation sequencing ([[NGS]]) leads to the discovery of many new genetic variants, as outlined by [https://www.researchgate.net/profile/Volker_Lauschke2 Volker Lauschke] ''et al.'' in [https://doi.org/10.2217/pgs-2016-0023 Requirements for comprehensive pharmacogenetic genotyping platforms]. |
| − | + | A challenge is how to evaluate the functional consequences of these mutations. A discussion of this is given by [https://www.researchgate.net/profile/Magnus_Ingelman-Sundberg Ingelman-Sundberg] ''et al.'' in the article [https://doi.org/10.1186/s40246-018-0157-3 Integrating rare genetic variants into pharmacogenetic drug response predictions]. Another article by the same authors is [https://doi.org/10.1016/j.ejps.2019.01.024 Prediction of drug response and adverse drug reactions: From twin studies to Next Generation Sequencing] | |
| + | |||
| + | ===Description of problem=== | ||
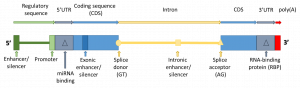
| + | The effects of mutations depend on the region in which they are encountered<ref>A good description is given by [https://doi.org/10.3389/fphar.2018.01437 Zouh ''et al.'']</ref>. | ||
| + | |||
| + | [[File:gene_outline.png|thumb|The effect of a mutation depends on the region where it is found. ]] | ||
| + | |||
| + | Disease causing mutations are often shared by a very small number of people due to evolutionary pressure. For genes involved in drug transport and metabolization, the evolutionary pressure is much weaker, and therefore the variants may be common. | ||
| + | |||
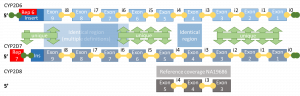
| + | An additional difficulty with determining genetic effect is for larger structural variants caused by copy number variations, pseudogenes or repeated regions. A good example of this is CYP2D6. | ||
| + | |||
| + | [[File:cyp2d6_outline.png|thumb|The effect of variants is also influenced by pseudogenes and repeated regions, as shown here for CYP2D6]] | ||
| + | |||
| + | ===Computational methods=== | ||
| + | A review of computational methods for PGx interpretation of NGS data has been written by [https://doi.org/10.3389/fphar.2018.01437 Yitian Zhou]. Zhou ''et al.'' also proposes a tool that combines several such methods, [https://www.nature.com/articles/s41397-018-0044-2 An optimized prediction framework to assess the functional impact of pharmacogenetic variants]. | ||
| + | |||
| + | ===Experimental methods=== | ||
| + | Ingelman-Sundberg ''et al.'' also propose a high-throughput ''in vitro'' method for gene function evaluation in [https://doi.org/10.2217/pgs-2018-0096 Human liver spheroids in chemically defined conditions for studies of gene–drug, drug–drug and disease–drug interactions]. This idea is the basis of the company [http://www.hepapredict.com/technology HepaPredict AB]. | ||
Latest revision as of 12:28, 27 February 2019
Next generation sequencing (NGS) leads to the discovery of many new genetic variants, as outlined by Volker Lauschke et al. in Requirements for comprehensive pharmacogenetic genotyping platforms.
A challenge is how to evaluate the functional consequences of these mutations. A discussion of this is given by Ingelman-Sundberg et al. in the article Integrating rare genetic variants into pharmacogenetic drug response predictions. Another article by the same authors is Prediction of drug response and adverse drug reactions: From twin studies to Next Generation Sequencing
Description of problem
The effects of mutations depend on the region in which they are encountered[1].
Disease causing mutations are often shared by a very small number of people due to evolutionary pressure. For genes involved in drug transport and metabolization, the evolutionary pressure is much weaker, and therefore the variants may be common.
An additional difficulty with determining genetic effect is for larger structural variants caused by copy number variations, pseudogenes or repeated regions. A good example of this is CYP2D6.
Computational methods
A review of computational methods for PGx interpretation of NGS data has been written by Yitian Zhou. Zhou et al. also proposes a tool that combines several such methods, An optimized prediction framework to assess the functional impact of pharmacogenetic variants.
Experimental methods
Ingelman-Sundberg et al. also propose a high-throughput in vitro method for gene function evaluation in Human liver spheroids in chemically defined conditions for studies of gene–drug, drug–drug and disease–drug interactions. This idea is the basis of the company HepaPredict AB.
- ↑ A good description is given by Zouh et al.